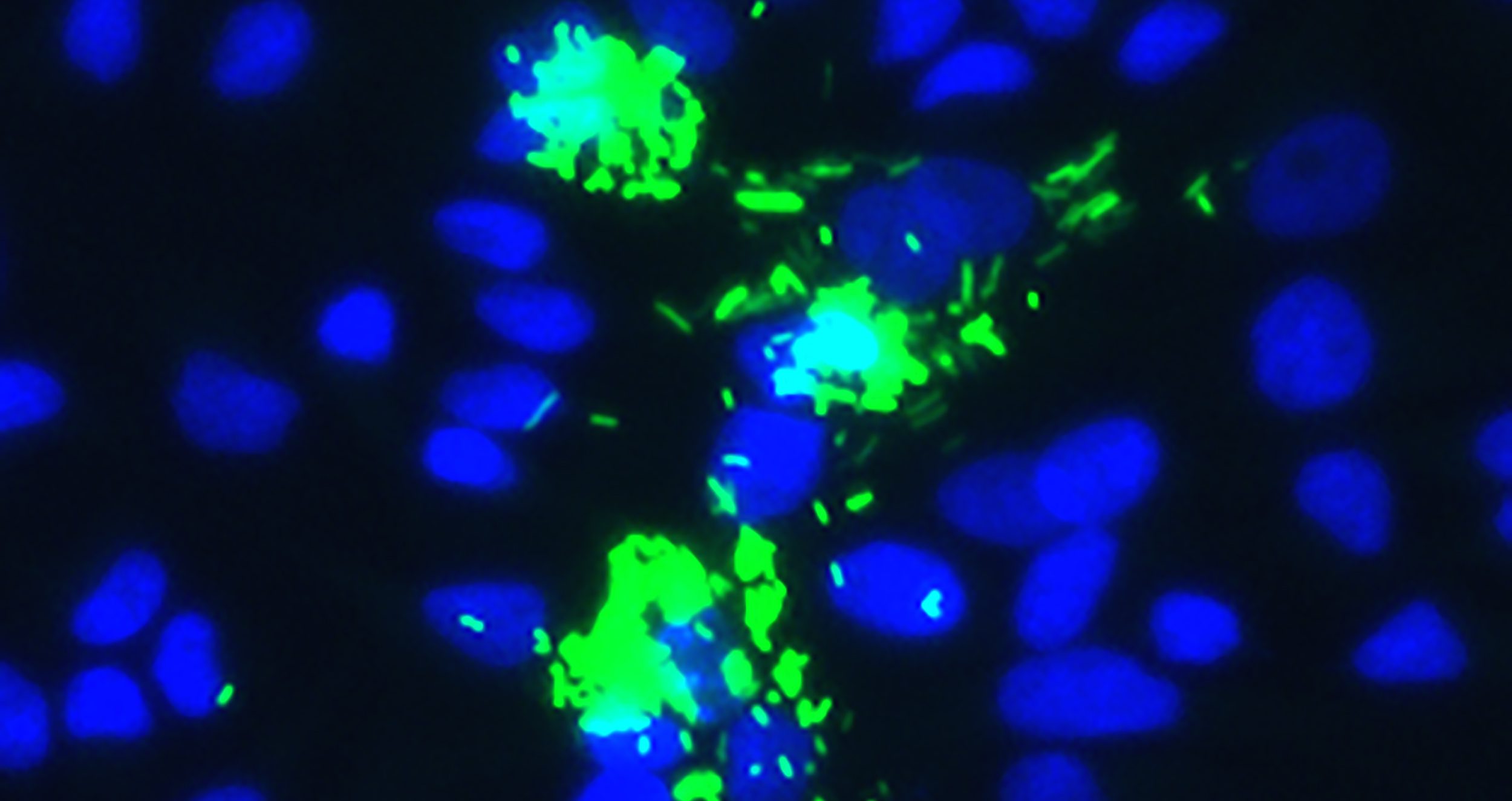
Shigella species invade epithelial cells in the gut using a type 3 secretion system (T3SS), which injects bacterial effector proteins into the host cell cytosol. Certain effector proteins permit invasion of the cell and subsequent lysis of the bacteria-containing vacuole. Other bacterial effector proteins manipulate host cell processes, promoting the survival and replication of the invading bacteria. We investigate the functions of these bacterial proteins during Shigella infection, endeavoring to reveal how they subvert hosts defenses.
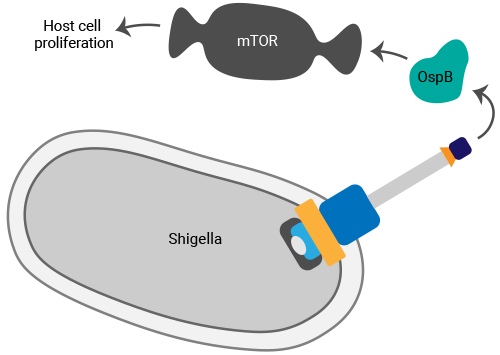
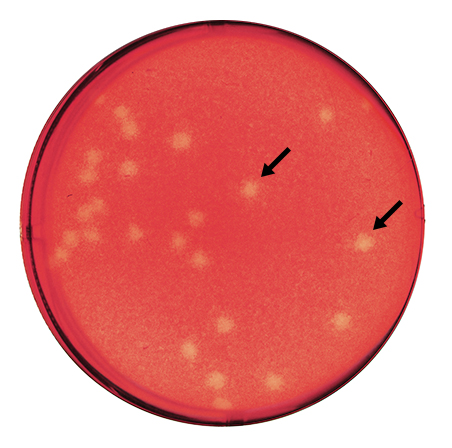
A key focus of our current research is on the spread of Shigella to neighboring cells. We found that one effector, OspB, manipulates mTOR signaling in cells in ways that promote cellular proliferation. In tissue culture, Shigella infection results in the death of epithelial cells, and its spread can be monitored through analysis of plaques formed in the cell monolayer. The increase in proliferation of infected host cells stimulated by OspB restricts plaque size as there are more adjacent cells to which the bacteria can spread. This likely increases the replicative niche of the pathogen, rendering it more difficult for the host to eliminate.
Future research will include investigations into the molecular mechanisms of other effector proteins, both of Shigella and of other gram-negative bacterial pathogens.